Presented at the Sixth Topical Meeting on Emergency Preparedness and Response American Nuclear Society,
San Francisco, April 22-25, 1997.
APPLICATION OF ADORA TO DISPERSION MODELING OF
INSTANTANEOUSLY RELEASED REACTING CHEMICALS
X. John Zhang, N. Albert Moussa, Daniel E. Groszmann, Michael C. Masonjones
BlazeTech Corporation, 24 Thorndike Street, Cambridge, MA 02141, (617) 661-0700
SUMMARY
An advanced source characterization and dispersion modeling system, ADORA (Atmospheric Dispersion Of Reacting Agents), for instantaneously released reacting chemicals has been developed. The model formulation includes the complex interactions of plume/cloud physics, chemical reactions, and thermodynamic transformations. A number of unique features are incorporated into the models such as constrained thermochemical equilibrium for high temperature reactions and simple global reaction equation for low temperature reactions; a reacting buoyant puff rise model for instantaneous releases; and a procedure for treating alternative behavior of puff motion, including lift-off from ground. ADORA is applied to three case studies: a reacting release of explosive materials, an accidental reacting spill of uranium hexafluoride (UF6), and a parametric study on the size of hydrocarbon fireballs. Excellent agreement is obtained in comparing model results with available experimental measurements.
I. INTRODUCTION
Planned or accidental releases frequently involve materials that react with ambient air. Chemical species are transformed and large amounts of reaction heat are produced. For example, open burn and open detonation are used in demilitarization to destroy unwanted materials. These activities involve both the initial explosive reactions as well as the succeeding reactions of explosion products with ambient air. Another example is the accidental release of industrial materials, such as uranium hexafluoride (UF6)1. These materials, upon release, will evaporate or sublimate into their corresponding vapor phase and react with water vapor in the air. Section 112(r) of Title III of the Clean Air Act Amendment (1990) requires a hazard assessment of the potential effects of accidental releases, as well as a plan of action for an emergency response. Consequently, an accurate modeling of these scenarios is greatly needed.
Current treatments assume that all the reactions finish immediately after the release. With an amount of air entrained based on a stoichiometric ratio or an empirical relation, the temperature, reaction products, and their amounts are calculated. The puff rise and subsequent passive dispersion are then obtained without involving further chemical reactions. While being simple and including some effects of the reactions, these treatments have several shortcomings: (1) When complex reactions (multiple steps and multiple components) are involved, it is difficult to determine a priori the amount of air that participates in the reactions; (2) The reaction products and their amounts are calculated without considering the time-dependent nature of air entrainment; (3) The concentration modeling at receptors close to the source may not be proper, since the reacting puff may pass by before the reactions are complete; and (4) An accurate treatment of instantaneously released buoyant puff rise is not used.
II. DESCRIPTION OF TWO TYPICAL SCENARIOS
Release of materials that involve chemical reactions can be generally divided into two categories: explosive release and reacting spill. The former is described below with an example of open burn and open detonation (OBOD), and the latter by an accidental release of UF6.
Demilitarization agencies frequently destroy unwanted munitions. A favorite method is by OBOD. A typical release scenario is as follows. During the explosion, the pressure and temperature of the puff (which is composed mainly of explosive products) reach a maximum. The puff then quickly expands and its pressure drops to ambient. A large amount of reaction heat is generated that raises the puff to a high altitude. Along its rise trajectory, the puff entrains ambient air and the reaction continues. After the puff reaches its final height, passive dispersion due to atmospheric turbulence takes over. The quantities of interest are the products formed and their concentration distribution in both space and time. Most observations reveal that a fireball tail is attached to the fireball. Even though the species concentration in the tail may be low, it can still be of importance since it is closer to the ground.
Industrial accidental releases of chemicals such as UF6 may experience the following stages. A fast flash is first encountered upon release, since most of these chemicals are stored as saturated liquids or solids with pressures higher than the ambient. A certain amount of liquid or solid is transformed to vapor as the cloud pressure drops to ambient. As more material vapor and water vapor become available through evaporation and entrainment, significant chemical reaction occurs. The initially spreading dense cloud may become buoyant and lift off from the ground, the final puff rise height being strongly affected by the reaction heat produced. Note that the puff lift-off may significantly reduce the toxic material concentration at ground.
These two cases show that the identities of toxic chemical species in the cloud and their maximum ground concentrations are strongly related to chemical reactions. Therefore, proper inclusion of reaction effects is critical to the dispersion modeling of chemical materials.
III. BRIEF MODEL DESCRIPTIONS
ADORA is a new generation model which includes exothermic/endothermic chemical reactions, multiphase and multicomponent thermodynamic transformations, and buoyant/dense/neutral puff behavior for an instantaneous release. More than 1600 common industrial chemicals and phases are in the database for explosive release and ten reaction equations are included for spill release.
ADORA models high temperature explosive release by treating the reactions as constrained thermochemical quasi-equilibrium. Low temperature spills are modeled using available global reaction equations. The interaction of three important components are included. They are:
(A) Puff/Cloud Physics This consists of buoyant puff rise, dense cloud spreading, lift-off, and passive dispersion. The released puff may experience a progression of stages, from being initially denser than air to lighter than air to neutral, and therefore may display different transport and dispersion characteristics.
(B) Chemical Reactions Toxic species may be destroyed or formed along with the release of reaction heat. The reaction rate is determined by the entrainment rate.
(C) Thermodynamic Transformation Most chemicals released may be in the form of liquid or solid phase. The time-dependent phase change, due to evaporation or sublimation, is calculated along with its effect on the puff density and temperature.
The description of these three modules for the explosive and spill scenarios are summarized in Table 1, where the Propellant Evaluation Program2 (PEP) is a computer code for calculating high-temperature thermodynamic properties and performance characteristics of propellant systems. In the modeling of explosive releases, the chemical kinetic reaction rates are not available in most cases. As a quasi-equilibrium approximation, PEP is applied to calculate the chemical compositions at each time step in a mixture with entrained air. This is valid when the mixture temperature is high and the reactions are fast. As more and more cool air is entrained in, the mixture temperature drops, some of the product species may disappear unrealistically, and the equilibrium approximation becomes inaccurate. A rationale is developed for allowing the amounts of individual species to be frozen at certain specified temperatures where the approximation starts to break down. Most frequently encountered frozen species and their corresponding temperatures are obtained, based on theoretical considerations with empirical validations. Other effects, not specifically mentioned above, such as radiative heat loss, ground heat transfer, wind shear, particle deposition, averaging time and concentration fluctuations are also included in the model.
Table 1. Three modules for modeling explosive release and reacting spill
|
IV. SAMPLE MODELING RESULTS
ADORA is applied to the dispersion modeling of three cases. The release conditions and the modeling results are summarized below.
A. Explosive Release
An explosion of magnesium [Mg], teflon [(C2F4)n], viton A [(C4H3F5)n], and paraffinic Oil [C12H22], with total mass of 88.71 kg, is released into the atmosphere. Summaries of input and output parameters are shown in Figure 1. In the products, nitrogen monoxide (NO), nitrogen dioxide (NO2), carbon monoxide, and hydrogen fluoride (HF) and magnesium oxide (MgO) are frozen at temperatures 1100K, 1700K, 2100K, 1100K, and 650K, respectively. A list of chemical species in the fireball, as well as in the tail at the final rise height, is shown. The tail effect is significant near the release point. The cloud reaches an equilibrium height of 490 m at a downwind distance of 356 m after 1.7 minutes with a diameter of 208 m. Table 2 presents the comparison of emission factors of various chemical species in the cloud with field test measurements3. The emission factor is defined as the ratio of mass of chemical products at the final rise height to the mass of the released chemicals.
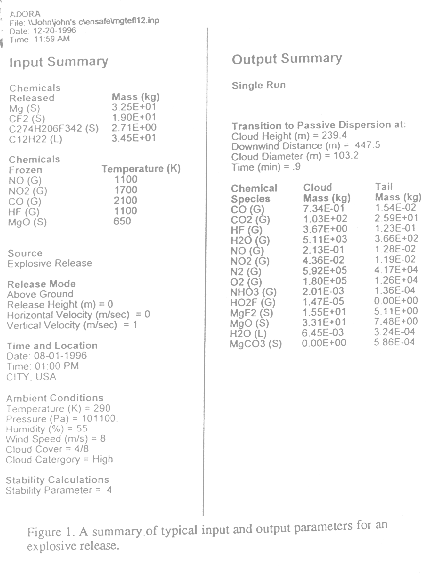 |
Table 2. Comparison of Emission Factors (mg/g), Observed//Predicted, Dugway Study
|
||||||||||||||||||||||||||||||||||||||||||||||||
B. Reacting Spill
In this case, we consider the instantaneous release of 1000 kg of saturated liquid UF6 at a storage temperature of 380K. Immediately after release, a flash occurs resulting in an initial mixture with only solid and vapor UF6. The solid UF6 sublimates while the vapor UF6 reacts with the moisture in the air. The ambient wind is 1 m/s measured at a 10 m height with a standard deviation in direction of 19 degrees. The ambient and ground temperatures are 295K; relative humidity is 80 percent; roughness length is 3 cm. Modeling results are shown in Figure 2.
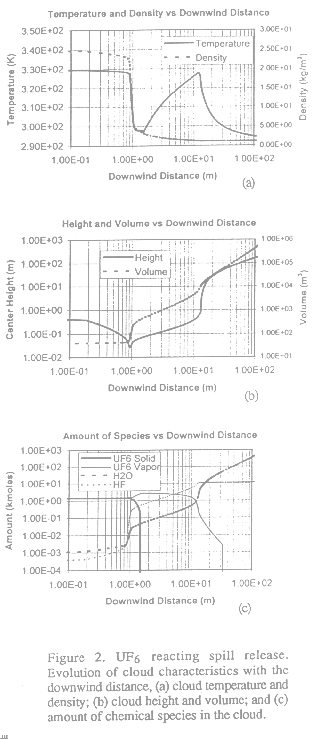 |
The first two plots present the variations of the cloud temperature, density, volume and height as the cloud drifts. The third plot shows the evolution of the amount of UF6 solid, UF6 vapor, H2O, and reaction product HF versus downwind distance. The solid UF6 is fully sublimated at 1.5 m. Before this point, not much reaction has taken place. The puff lifts off the ground at 13 m due to the large amount of reaction heat released. Afterwards, the buoyant puff experiences a faster rise and the chemical reaction stops at 33 m. The cloud temperature then drops quickly and the puff reaches an equilibrium height of 204 m at a downwind distance of 127 m. After this point, the ambient turbulence dominates the puff motion and the dispersion is treated as passive (not shown in Figure 2).
C. Hydrocarbon Fireballs
Figure 3 shows the modeling results of reacting hydrocarbon fireballs in the air. Fireball diameter versus the combustion energy of the released chemicals is presented. Measurements (open symbols) are presented for a number of fuels and propellants, ranging from small to large scale tests to a few accidents. Since the conditions for the measured fireball diameters were not clearly defined, considerable scattering of the data points is evident. We calculated the fireball diameters corresponding to two times after release: (1) when the reacting puff reached its maximum temperature; and (2) when all fuels have been consumed. They are shown as solid circle and square symbols, respectively. The choice of these two times reflects part of the uncertainty in measuring the diameter. The model results fall on the mean of the measured test data. These results compare very well with both field and laboratory test results over seven orders of magnitude in energy release.
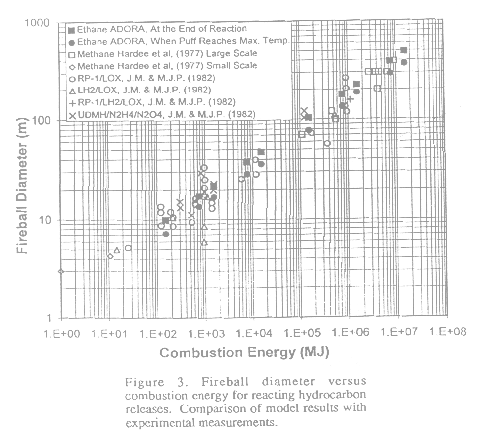 |
V. CONCLUSION
A source characterization and atmospheric dispersion model that predicts the product species and their concentrations has been developed. The model includes the interactions of three interrelated modules: physical dispersion, chemical reaction, and thermodynamic transformations. ADORA is also equipped with a GUI (Graphic User Interface) and can be run on a PC 486+ with 16 MB RAM. The model bridges the gap between source specification and meteorological dispersion by careful consideration of near-source effects, including the product formation and exothermic heat release. Thus, unnecessary conservatism in designing and planning for storage or test facilities can be relaxed.
This model has a wide range of applications such as: (a) reaction of halogenated compounds with moisture; (b) hydrocarbon combustion; (c) chemical releases triggered by explosives. The model can be used to meet the regulations requirements, e.g., CAA 112(r), RCRA Part B, and OSHA PSM (29 CFR 1910) for hazard/risk/safety assessment. To expand the potential application, we are interfacing ADORA with a Geographic Information System (GIS) and real time meteorological data input.
REFERENCES
1. Hanna S.R., Chang J.C. and Zhang X.J., "Modeling accidental releases to the atmosphere of a dense reactive chemical (Uranium Hexafluoride)," Atmospheric Environment (To appear).
2. Cruise D. R., Theoretical Computations of Equilibrium Composition, Thermodynamic Properties, and Performance Characteristics of Propellant Systems. NWC TP 6037, Naval Weapons Center, China Lake, CA 93555-6001, (1991).
3. Headquarters U.S. Army Armament, Munitions and Chemical Command, Development of Methodology and Technology for Identifying and Quantifying Emission Products from Open Burning and Open Detonation Thermal Treatment Methods, (1992).